By Dan Hosseinzadeh,
CEO, Pathcore and
Co-Chair, DICOM Working Group for Pathology (WG-26)
A recent article from the National Academy of Medicine: Procuring Interoperability: Achieving High-Quality, Connected, and Person-Centered Care highlights the results of adopting standards for true interoperability: more patient-centric and high quality care. DICOM is the universal standard for interoperability in medical imaging - we wrote a blog about why it's important that you can read here.
Following on this topic, here we explore how DICOM’s Pathology Working Group (WG-26) is pushing the boundaries of interoperability through a series of DICOM Digital Pathology Connectathons. In the past year, WG-26 organized four DICOM Digital Pathology Connectathon events at the following pathology conferences:
Pathology Visions 2017, San Diego (PV17),
Pathology Informatics Summit 2018, Pittsburgh (PI18),
European Congress of Digital Pathology 2018, Helsinki (ECDP18), and
Pathology Visions 2018, San Diego (PV18).

To increase adoption of the universal medical imaging format DICOM for pathology, WG-26 designed a series of DICOM Digital Pathology Connectathons with several goals in mind. These objectives, and how they were achieved through the connectathons will be discussed in this article.
Objective 1: Demonstrate the benefits of DICOM for whole slide imaging to the pathology community
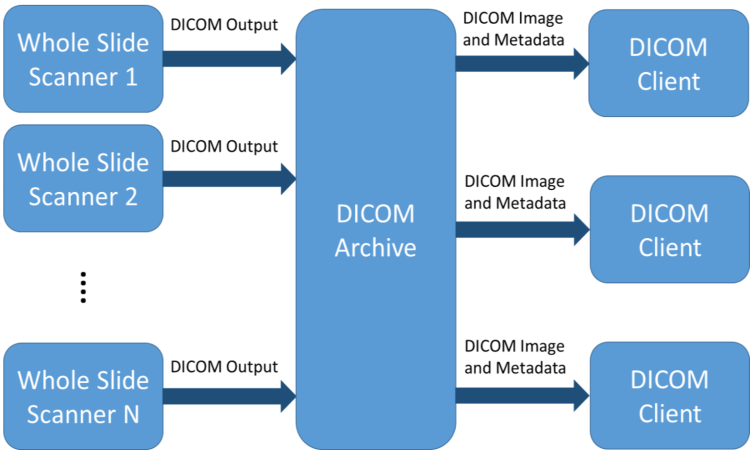
The connectathons provided an opportunity for those interested in DICOM to experience it first-hand through a mix of real-time experiments and educational events. The live demonstrations showed how hardware and software solutions from different vendors could successfully exchange data using the DICOM standard. The workflow for the interoperability challenges is shown in Figure 1. Multiple scanner vendors acquired whole slide images and generated DICOM image outputs, which were then transferred and stored in a DICOM archival solution. Finally, multiple vendors retrieved the DICOM images from a DICOM archive for the purposes of viewing and analysis (DICOM Client).
Educational opportunities were provided to attendees through hour long discussion panels at each event, where vendors and experts discussed what DICOM has to offer, their vision for DICOM, and answered questions about interoperability. Attendees ranged from clinicians, pathologists, engineers and computer scientists. They had the opportunity to engage directly with vendors through the showcases to get more in-depth answers to some of their questions about DICOM and interoperability.
“In the last decade, several vendors have produced increasingly capable automated, high-speed whole slide imagers. The quality of the images produced is of diagnostic quality and with the viewing software; it is possible to have annotations and clinical metadata presented with the image. The DICOM Connectathons that have been conducted in the last 12 months have clearly demonstrated that it is possible to achieve interoperability between several different scanning devices, viewing software and image management systems. These efforts have already paved the way for increasing adoption of whole slide imaging for multiple clinical applications.” - Anil Parwani, M.D., Ph.D., M.B.A., Wexner Medical Center
Objective 2 & 3: Engage vendors to further develop DICOM capabilities in their products & provide a mechanism for experimenting with interoperability
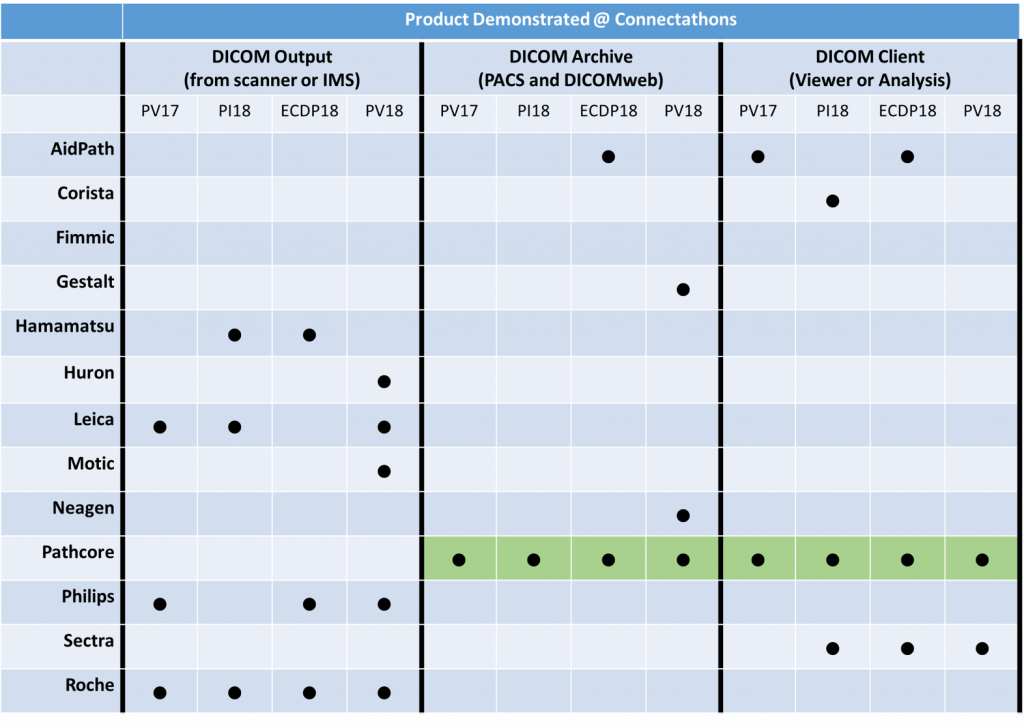
Another major motivation for the connectathons is to bolster the capacity of solution providers to offer products that are DICOM compliant. Vendors prepare months before-hand, and work with other vendors to showcase their DICOM-based interoperability. Through each iteration, participating vendors test and improve their DICOM capabilities for digital pathology. Table 1 shows the participants involved in the past four connectathons, and several vendors can now claim DICOM conformance to some level. Many other vendors have integrated DICOM functionality into their R&D (pre-commercial) pipelines, as well. With each iteration of the event, we have engaged more participants (mostly vendors, but also some academic groups) for a total of 13 different participants, who continue to improve their DICOM capabilities incrementally.
Objective 4: Identify deficiencies, if any, in DICOM for WSI and expand the standard
As exhibited by the connectathons, which provided a venue to test the DICOM standard, it was indeed shown that DICOM is a suitable solution for WSI since interoperability was exhibited across vendors. During these events, DICOM-based viewers were shown to be fast and faithful in the way they render images vs. the proprietary image format counterparts. For instance, it was clear that the performance of the DICOM viewers were comparable with the proprietary viewers- which is expected since the underlying data remains unchanged in DICOM format. We also saw that several viewers were able to render color accurately, just like the proprietary viewers.
Although promising, through the connectathons and regular meetings, WG-26 has identified a series of issues (edge cases) with the DICOM standard that pose challenges for pathology. As a group, we are currently endeavouring to solve these challenges, leading to at least twelve different enhancements to the standard (either proposed or accepted). These improvements are listed in Table 2. Some of the improvements are: a) related to minor details that correct the consistency of the DICOM standard for WSI such as CP-1740; b) performance tweaks that ensure DICOM viewers can be as performant as the proprietary viewers such as CP-1713; c) color management improvements such as CP-1804 that ensure color consistency can be precisely managed for web-based DICOM viewers; and d) several other minor improvements are described in Table 2 below.
In order for these changes to be accepted, all members of the WG-26 community, which includes digital pathology vendors, clinicians, academics, and other affiliate groups, must source and vet these improvements. In addition to peer review and vettings within WG-26, the DICOM standards Committee (Working Group 6) always weighs in and approves any proposals generated from the other DICOM Working Groups. This ensures that all the improvements are validated prior to becoming part of the standard and not skewed by the preferences of any particular vendor.

Objective 5: Enhance the technical scope of the demonstration in each iteration
The final objective speaks to the fact that the Connectathons must evolve and grow so that eventually multiple clinical workflows can be demonstrated. To date, we’ve focussed on ensuring free and simple data exchange using a common standard for digital pathology. We’ve largely achieved this goal for pixel data. Going forward, WG-26 is working to expand this capability by defining the standard for metadata exchange with the LIS in coordination with the IHE PaLM leading to the development of an IHE integration profile specifically for digital pathology. Once achieved, the scope of the connectathons will be expanded to include these specifications as well. Another enhancement envisioned for the Connectathons is to test, integrate and promote the use of DICOM with image analysis software vendors. These two improvements will complete the interoperability circle for exchanging pixel data, metadata and analysis data using DICOM - an idea that is worth working for.
“The DICOM Connectathons are the next best thing to have happened in Digital Pathology since FDA approval of WSI for primary diagnosis. It is encouraging to see different vendors working together to make their systems plug & play in our labs. This affirms that we are on the right path ahead for WSI”. - Liron Pantanowitz, MD, UPMC